Shutting down a gene called PRMT5 stopped metastatic estrogen receptor-positive (ER+) breast cancer cells from growing after they acquired resistance to a standard therapy known as CDK4/6 inhibitors, UT Southwestern Medical Center researchers have shown in a recent study.
Their findings, published in Nature Communications, could lead to new strategies to treat metastatic ER+ breast cancer, the most common subtype that is responsible each year for the most deaths from this disease.
“Combining an anti-estrogen therapy with a drug that inhibits PRMT5 could eventually offer a new way to treat patients resistant to CDK4/6 inhibitors, who often have few options left,” said Chang-Ching “Albert” Lin, Ph.D., Assistant Professor in the Harold C. Simmons Comprehensive Cancer Center and in the Department of Internal Medicine at UT Southwestern.
Dr. Lin co-led the study with Carlos L. Arteaga, M.D., Professor and Director of the Simmons Cancer Center and Associate Dean of Oncology Programs at UTSW. Dr. Lin is a former postdoctoral researcher in the Arteaga Lab.
Nearly 700,000 women around the globe die from breast cancer each year, mainly from a subtype of the disease that bears estrogen receptors (ERs) and metastasizes, or spreads, to other parts of the body. Doctors have increasingly treated metastatic ER+ breast cancers by combining drugs that degrade or block the estrogen receptors on breast cancer cells in combination with CDK4/6 inhibitors, which together inactivate proteins necessary for breast cancer cells to divide and multiply.
Although CDK4/6 inhibitors can block cancer progression and extend survival, they come with a drawback: Nearly universally, tumors stop responding to these drugs over time, leaving patients with few treatment options, Dr. Lin explained. Previous studies have shown that an increasing number of tumors resistant to CDK4/6 inhibitors have lost the function of a gene called RB1.
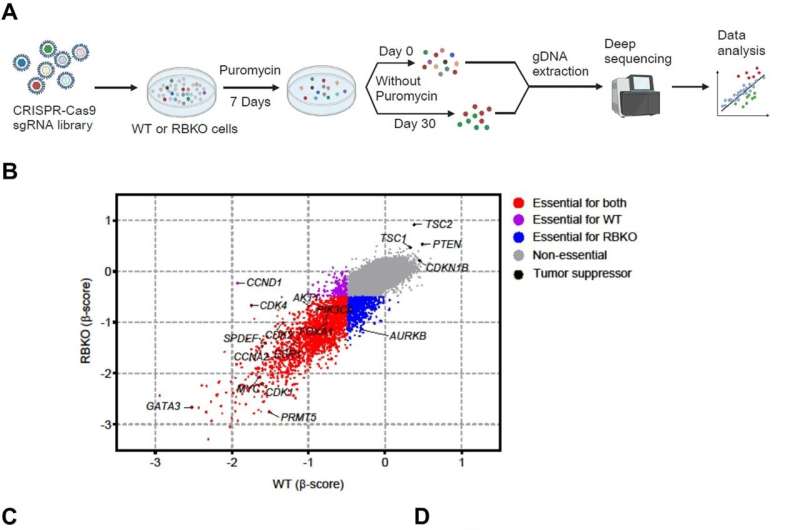
Searching for a new strategy to treat metastatic ER+ breast cancer resistant to CDK4/6 inhibitors, Drs. Lin and Arteaga and their colleagues used the CRISPR gene-editing tool on cells from two ER+ breast cancer lines to delete RB1—making them resistant to all three CDK4/6 inhibitors approved by the U.S. Food and Drug Administration.
They then screened more than 19,000 other genes, searching for those that might play key roles in cell survival and growth. Their investigation homed in on PRMT5, a gene already implicated in the progression of various cancer types, including breast, and for which pharmaceutical inhibitors are in development.
When the researchers reduced the amount of protein produced by PRMT5 with a genetic technique or administered a PRMT5 inhibitor in clinical development, the cells got stuck in a part of their cell cycle known as the G1-S transition, in which DNA is copied before cells divide.
Further investigation showed that PRMT5 helps regulate a bevy of genes involved in DNA replication. When the researchers administered the inhibitor along with a drug that degrades ERs in mice bearing human ER+ tumors with RB1 deletion, the dual treatment blocked these tumors from growing significantly better than either treatment alone, putting the animal models in partial remission.
Dr. Lin noted that he, Dr. Arteaga, and their colleagues hope to test this combination in patients with ER+ breast cancer in future clinical trials.
More information:
Chang-Ching Lin et al, PRMT5 is an actionable therapeutic target in CDK4/6 inhibitor-resistant ER+/RB-deficient breast cancer, Nature Communications (2024). DOI: 10.1038/s41467-024-46495-2
Citation:
Blocking gene may halt growth of breast cancer cells (2024, April 25)
blocking-gene-halt-growth-breast.html
.
. The content is provided for information purposes only.
