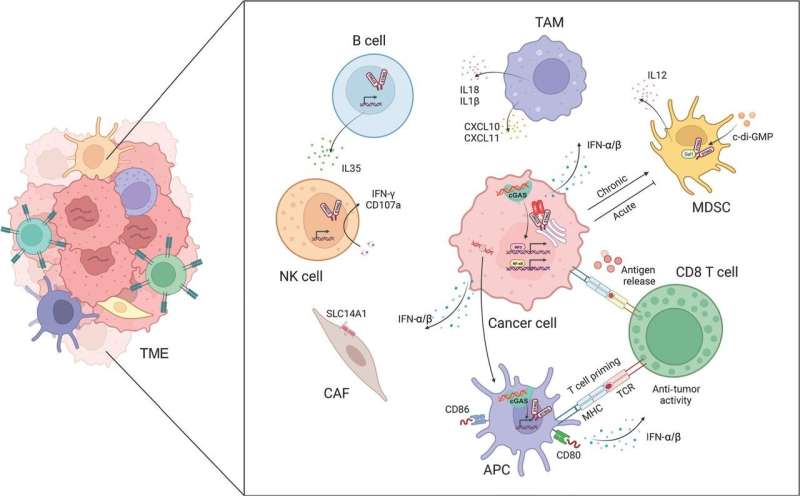
Cancer immunotherapy, which leverages the body’s immune system to target tumors, has emerged as a pivotal strategy in oncology. This paradigm shift from conventional treatments offers a more precise method to neutralize cancer cells. The cGAS-STING signaling pathway, a key element of the innate immune response, has risen as a promising avenue to amplify this therapeutic approach.
Unraveling the pathway’s role in immune system activation could yield more effective treatments and enhanced patient outcomes in the ongoing fight against cancer.
A collaborative review published in Cancer Biology & Medicine casts light on the cGAS-STING signaling pathway, highlighting its substantial potential for cancer immunotherapy. The review scrutinizes the complex dynamics between the human immune system and cancer cells, presenting a hopeful new direction for therapeutic intervention.
The core study behind this scientific milestone offers an exhaustive examination of the cGAS-STING signaling pathway, a fundamental component of the innate immune system that is crucial for the detection and response to cancerous cells.
The research team conducted a meticulous analysis of how the pathway’s activation can initiate a sequence of immune reactions, including the synthesis of type I interferons (IFN-?/?), which are vital for the development, specialization, and deployment of immune cells within the tumor microenvironment.
The team discovered that the presence of double-stranded DNA (dsDNA) from compromised or deceased cancer cells triggers the cGAS-STING pathway. This activation induces structural alterations in the STING protein, prompting its relocation from the endoplasmic reticulum to the Golgi apparatus, thereby initiating a signaling sequence.
This sequence culminates in the engagement and activation of essential kinases and transcription factors, such as TBK1 and IRF3, which drive the transcription of genes responsible for the production of type I interferons and other molecules that stimulate the immune system.
Dr. Ping Wang, a principal investigator in the study, says, “Our investigation into the cGAS-STING pathway, now recognized as a critical asset in cancer treatment, is unveiling new pathways to more effective and precise cancer therapies.”
The research findings carry profound implications for cancer treatment strategies, indicating that the activation of the cGAS-STING pathway could transform the landscape of immunotherapy. This insight could facilitate the creation of targeted therapies designed to boost immune responses against cancer. The promising results from clinical trials employing pharmacological agonists and combination treatments bode well for potential improvements in patient survival rates, pointing to an auspicious future for cancer treatment.
More information:
Jiawen Zhang et al, Emerging mechanisms and implications of cGAS-STING signaling in cancer immunotherapy strategies, Cancer Biology & Medicine (2024). DOI: 10.20892/j.issn.2095-3941.2023.0440
Citation:
Unlocking the immune system: cGAS-STING pathway offers new hope for cancer treatment (2024, April 30)
immune-cgas-pathway-cancer-treatment.html
.
. The content is provided for information purposes only.
