The human immunodeficiency virus (HIV) is a dangerous, hard-to-treat virus. It mutates rapidly. It has developed structures to shield itself from recognition and attack by antibodies and therapeutics.
HIV mainly targets T cells, which are immunological cells, weakening them and making it impossible for them to protect other body cells from infection. An HIV virion changes its form a little bit when it gets ready to enter a T cell. These occur on the so-called envelope protein of the virus, which is the surface protein that allows the virus to enter cells. Since envelope proteins are crucial to the virus’s ability to infect, they make excellent targets for medications or vaccinations.
The HIV envelope protein is “trimeric,” resembling a tripod-like flower with three “stem” portions—each called gp41—and three “petal” regions called gp120. To initiate the infection, each of the three gp120 proteins grabs on to a kind of receptor on the T cell called CD4. Once three CD4 receptors are secured by the three gp120 proteins, they expose sites that are recognized by a host co-receptor, and then a needle-like structure emerges from the stem regions of the “flower,” enabling the virus to infect and gain entry into the human cell.
But what if the envelope protein’s gp120 “petals” are only able to grab onto one or two CD4 receptors? Can the envelope protein still fully open up so the virus can infect the cell? Understanding this process could have significant implications for designing therapeutics. If researchers could prevent just one or two CD4 receptors from being grabbed by a gp120, would that be sufficient to thwart infection? To answer this open question, the team sought to image the envelope protein in these scenarios with only one or two CD4s bound.
“Structurally characterizing the intermediate envelope conformations is incredibly valuable to understand how HIV proteins work on a fundamental level,” says Dam.
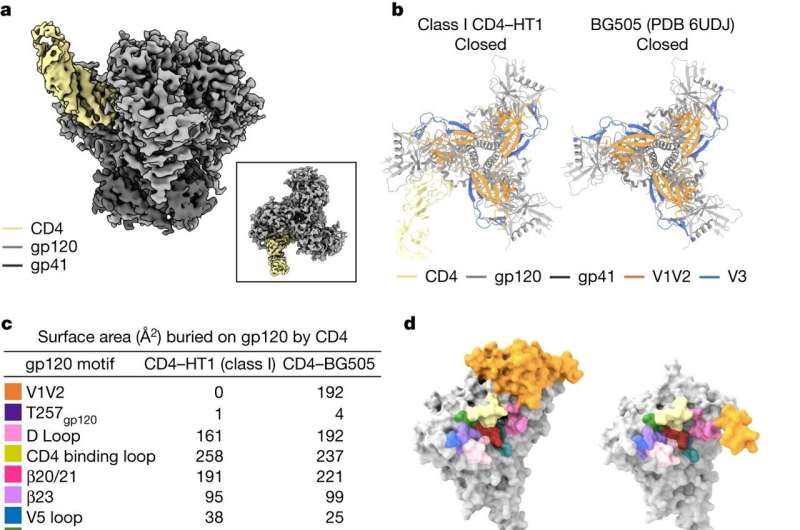
But imaging these structures is a challenge: Creating “heterotrimers,” or envelope proteins that bind only one or two CD4 receptors, is not easy to do in a test tube for biochemical reasons. Through an innovative engineering approach, the team was able to devise a protocol to create stable heterotrimers. Then, utilizing Fan’s expertise in a delicate procedure called cryo-electron microscopy, they were able to take images of the fragile heterotrimers’ structures bound to CD4 receptors.
The structures showed that if only one or two CD4 receptors are bound, the envelope protein is not able to fully open up and undergo the shape-shifting process associated with infection.
“One major question that comes out of this work is: Can envelope proteins that do not fully open up still facilitate infection?” says Dam.
The team then shared results with the laboratory of Walther Mothes at Yale University, which was conducting similar attempts to image heterotrimers. Sharing information between the two labs showed that the behavior of engineered heterotrimers free-floating in a test tube (an experimental setup where proteins are in solution or soluble as opposed to bound to viral membranes) is remarkably similar to how envelope proteins on the viral surface behave in a more “real world” infection scenario.
This is an important finding because soluble constructs are used as the basis of developing new therapeutics, and it is critical to know if they are accurately mimicking natural processes.
Structural biology research like this is not only important for studying HIV but many different kinds of viruses.
“We’ve learned so much from HIV,” says Dam. “When the COVID-19 pandemic started, we applied what we had learned from HIV to SARS-CoV-2.”
“The structures of these previously unknown, intermediate envelope conformations offer fascinating insights into the structural changes driven by receptor interactions before host and viral membrane fusion,” says Björkman. “Our research not only opens new avenues for exploring the complexities of HIV infection but also provides valuable insights that extend beyond therapeutic design, enhancing our overall understanding of viral dynamics.”
More information: Dam, KM.A. et al, Intermediate conformations of CD4-bound HIV-1 Env heterotrimers, Nature (2023). DOI: 10.1038/s41586-023-06639-8. www.nature.com/articles/s41586-023-06639-8
