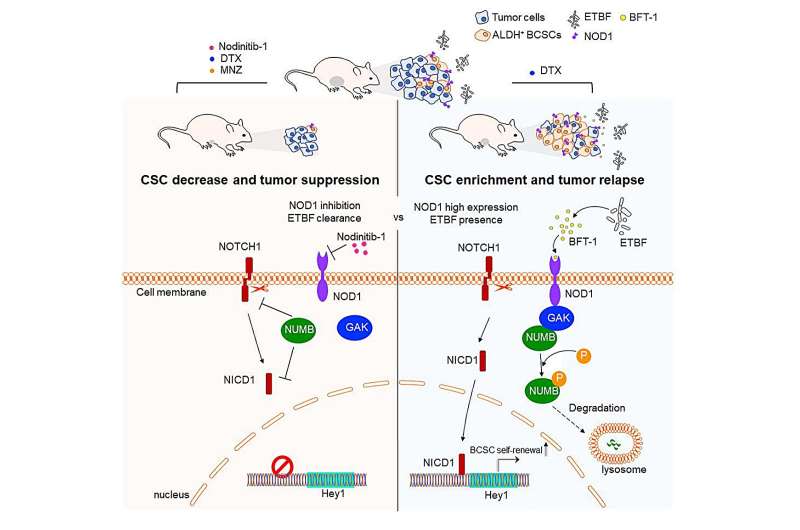
Tumor-resident microbiota in breast cancer promotes both the initiation and progression of cancer. However, the potential of targeting microbiota to enhance the efficacy of breast cancer treatment has not been comprehensively explored. In this study, researchers analyzed the microbial composition within breast tumors and identified a notable enrichment of ETBF in patients who exhibited resistance to taxane-based neoadjuvant chemotherapy.
The work titled “Microbiota enterotoxigenic Bacteroides fragilis-secreted BFT-1 promotes breast cancer cell stemness and chemoresistance through its functional receptor NOD1” was published in Protein & Cell.
Key findings from the study include:
- Even at low biomass levels, ETBF secretes a toxic protein, BFT-1, which enhances breast cancer cell stemness and chemoresistance.
- Mechanistic investigations revealed that BFT-1 directly binds to the NOD1, a protein highly expressed in the ALDH+ BCSCs. The interaction between BFT-1 and NOD1 leads to the stabilization of NOD1.
- NOD1 cooperated with G-associated kinase (GAK) to phosphorylate NUMB, leading to its lysosomal degradation. This process activated the NOTCH1-HEY1 signaling pathway, thereby increasing the number of BCSCs.
- Inhibition of NOD1 and clearance of ETBF significantly increase chemotherapy sensitivity by impairing breast cancer stem cells.
These findings suggest that inhibiting the NOD1 pathway and eliminating ETBF from the tumor microenvironment could potentially sensitize breast cancer cells to chemotherapy by impairing BCSCs.
The results highlight the importance of considering the microbiome as a factor in cancer treatment and represent a promising avenue for developing novel therapeutic strategies aimed at enhancing the efficacy of existing breast cancer treatments.
More information:
Wei Ma et al, Microbiota enterotoxigenic Bacteroides fragilis-secreted BFT-1 promotes breast cancer cell stemness and chemoresistance through its functional receptor NOD1, Protein & Cell (2024). DOI: 10.1093/procel/pwae005
Provided by
Higher Education Press
Citation:
Microbiota B. fragilis-secreted BFT-1 promotes breast cancer cell stemness and chemoresistance: Study (2024, April 25)
microbiota-fragilis-secreted-bft-breast.html
.
. The content is provided for information purposes only.
