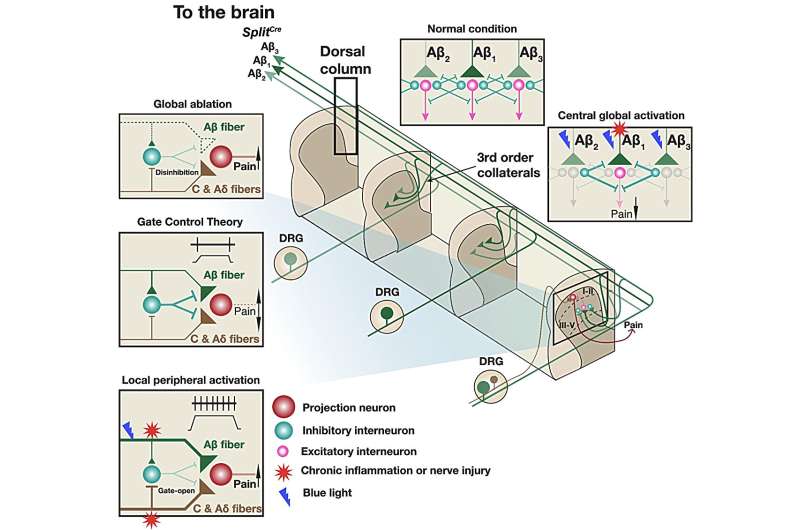
People often experience the phenomenon of injuring a hand and feeling relief from vigorously shaking it. The mechanism behind this effect is fairly well explained by the “gate control theory” of Melzack and Wall, but it is somewhat more complicated.
Shaking your hand modifies the pain signals in the spinal cord in the superficial dorsal horn area, which are transmitted by A? and C fibers. This area is activated by pain signals, but there are other connections responsible for sensing touch (A? mechanoreceptors) and they can block the pain signals at this location. Therefore, this crosstalk between touch and pain neurons finally decides the noxious or non-noxious output depending on the type and duration of injury.
Chronic pain and its relevance
Chronic pain is a catastrophic disorder of the nervous system affecting more than 30% people worldwide, creating a significant socioeconomic burden. A prominent symptom of chronic pain is mechanical hyperalgesia, or increased pain triggered by mechanical stimuli. At present, effective non-opioid treatment for mechanical hyperalgesia is limited, reflecting a knowledge gap in its underlying mechanisms.
Complicated role of A? low threshold mechanoreceptors in pain
A? low-threshold mechanoreceptors (A?-LTMRs) are large-diameter, highly myelinated and fast-conducting primary somatosensory neurons, which normally mediate tactile, discriminative touch and vibration sensation.
A?-LTMRs are further divided into rapidly adapting (RA) and slowly adapting (SA) types based on their firing patterns in response to a sustained mechanical stimulus. At baseline conditions, co-activation of touch- and pain-sensing neurons inhibits the flow of nociceptive information, as proposed in the “gate control theory of pain.”
Functions of A?-LTMRs in chronic pain have been of great interest but are controversial. On one hand, several studies suggested that A?-LTMRs may mediate mechanical hyperalgesia of chronic pain; some studies suggest that A?-LTMRs inhibit nociception even in chronic conditions. Rubbing or massaging a painful area of the skin, which presumably activates A?-LTMRs, attenuated pain in humans.
The spinal cord stimulator (SCS) and transcutaneous electrical nerve stimulation (TENS), which was developed based on the “gate control theory of pain” to target A?-LTMRs, are effective for treating chronic pain conditions, including neuropathic pain. Additional real-life therapy procedures, including massage therapy and electroacupuncture, presumably involve the activation of A?-LTMRs for their beneficial effects.
What may lead to the diverse role of A? low threshold mechanoreceptors in pain?
When comparing different studies and their results, we noticed two factors that likely contribute to the diverse outcomes of A?-LTMRs in transmitting mechanical hyperalgesia or in its alleviation.
The first consideration is the specificity of A?-LTMR manipulation and the functional readouts. Though pharmacological and mechanical/electrical stimuli can inhibit or activate A?-LTMRs, other types of nerve fibers are likely simultaneously affected as well. For genetic and optogenetic manipulations, few available mouse genetic tools are selective or show high preference for A?-LTMRs. In addition, it has been challenging to differentiate touch and nociception-associated reflex behavioral responses in animal studies.
The second consideration is whether A?-LTMRs are manipulated locally at the area affected by chronic pain or in a broad manner, including the unaffected A?-LTMRs, which may generate different sensory and behavioral outcomes.
To provide novel insight into this question, we used tools with improved specificity, including intersectional mouse genetics, opto-tagged electrophysiological recordings (in collaboration with Dr. Jianguo Gu’s lab at University of Alabama at Birmingham), and optogenetic activation of A?-LTMRs, and performed a battery of behavioral tests to probe mouse mechanical and thermal sensations when A?-LTMRs were manipulated locally or globally.
We also took advantage of a high-speed imaging method to differentiate touch-related non-nociceptive behavior from nocifensive behaviors. Our study is published in the journal Nature Communications.
Major outcomes of the study
- Mice with global ablation of SplitCre-A?-LTMRs showed decreased sensitivity to gentle mechanical forces, increased mechanical nociception at baseline condition, and increased mechanical hyperalgesia in a chronic inflammatory pain model, suggesting that A?-LTMRs function to inhibit mechanical nociception and mechanical hyperalgesia.
- Thermosensation was not affected by A?-LTMR manipulation, indicating that these afferents functioned in a modality-specific manner.
- In reverse experiments, local optical activation of SplitCre-A?-LTMRs at the hind paw triggered nocifensive responses in chronic inflammatory and neuropathic pain models. This suggests that activities of locally affected A?-LTMRs lead to nociception, likely contributing to mechanical hyperalgesia of chronic nociception.
- Rapidly adapting mechanoreceptors in the glabrous skin following tissue inflammation fired more numbers of action potentials (displaying a slowly adapting-like property).
- Global activation of A?-LTMRs at the dorsal column alleviated mechanical hyperalgesia in a chronic inflammatory pain model.
Conclusion
Together, the results of our study establish a model that A?-LTMRs play globally inhibitory but locally promoting roles for mechanical hyperalgesia. This model suggests a strategy: global activation plus local inhibition of A?-LTMRs for treating mechanical hyperalgesia of chronic pain. Some transcutaneous electrical nerve stimulation (TENS) strategies aim to target peripheral A?-LTMRs 2-3 dermatomes away from the injury site; their effects in alleviating pain were supported by several clinic trials. These practices and effects also align well with this study’s results and model.
Finally, this model proposes a potentially more effective compound strategy to target A?-LTMRs for treating mechanical hyperalgesia and chronic pain: activating them globally or three to four dermatomes away from the injury site and combining with local inhibition of the affected A?-LTMRs.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about Science X Dialog and how to participate.
More information:
Mayank Gautam et al, Distinct local and global functions of mouse A? low-threshold mechanoreceptors in mechanical nociception, Nature Communications (2024). DOI: 10.1038/s41467-024-47245-0
Mayank Gautam is a Research Associate in Department of Neuroscience at Perelman School of Medicine of University of Pennsylvania in Wenqin Luo’s Lab. Gautam has research experience of more than 10 years in the field of Neuroscience/somatosensation. Gautam is the first author of this study, which found the distinct local and global functions of touch neurons in inflammatory pain condition in mice.
Citation:
Neuroscientists report a distinct role of touch receptors in treating chronic pain (2024, April 23)
neuroscientists-distinct-role-receptors-chronic.html
.
. The content is provided for information purposes only.
