A team of researchers led by Dr. Hirotsugu Oda at the University of Cologne’s CECAD Cluster of Excellence for Aging Research has discovered the role a specific protein complex plays in certain forms of immune dysregulation. The result may lead to new therapeutic approaches aimed at reducing autoinflammation and “repairing” the immune systems of patients who suffer from a genetic dysfunction of this protein complex.
The study “Biallelic human SHARPIN loss of function induces autoinflammation and immunodeficiency” has been published in the journal Nature Immunology.
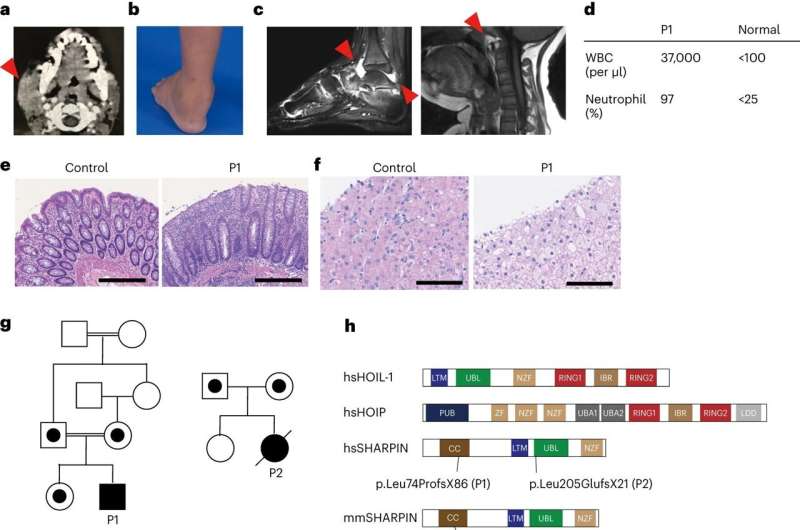
LUBAC, the linear ubiquitin assembly complex comprising the proteins HOIP, HOIL-1, and SHARPIN, has long been recognized for its critical role in maintaining immune homeostasis. Previous studies conducted on mouse models have elucidated the profound consequences of SHARPIN loss, leading to severe dermatitis due to excessive cell death of skin cells. However, the specific implications of SHARPIN deficiency in human health have remained elusive until now.
The research team reports for the first time on two human individuals with SHARPIN deficiency who exhibit symptoms of autoinflammation and immunodeficiency, but unexpectedly do not manifest dermatological issues as indicated by the relevant animal model.
Upon further investigation, these individuals displayed a pathway that is crucial for immune response, the so-called compromised canonical NF-?B response. They also showed increased susceptibility to cell death mediated by tumor necrosis factor (TNF) superfamily members. Treatment of one SHARPIN-deficient individual with anti-TNF therapies, which specifically suppress TNF-induced cell death, resulted in the complete resolution of autoinflammation at the cellular level and in clinical presentation.
The study shows that excessive and unchecked cell death plays a critical role in human genetic inflammatory diseases. Oda’s team added SHARPIN deficiency as a novel member of human genetic inflammatory diseases, which they propose to name “inborn errors of cell death.”
Safeguarding against immune dysregulation
The study was initiated at Dr. Dan Kastner’s lab at the National Institutes of Health (NIH) in the U.S.. The scientists there had the opportunity to see one patient with childhood-onset unexplained fever episodes, arthritis, colitis and immunodeficiency.
Upon obtaining informed consent, they performed exome sequencing of the patient and the family members and discovered that the patient had a deleterious genetic variant in the SHARPIN gene, leading to undetectable levels of SHARPIN protein. They further revealed that the patient cells show an increased propensity for cell death both in cell culture dishes and in patient biopsies.
The team further observed that the development of lymphoid germinal centers—the specialized microstructures in adenoids which are critical for the maturation of the B cells of our immune system, and thus antibody production—was markedly diminished due to increased B cell death. These findings account for the immunodeficiency in patients and highlight the pivotal role of LUBAC in immune homeostasis in humans.
“Our research underscores the critical importance of LUBAC in safeguarding against immune dysregulation. By elucidating the molecular mechanisms underlying LUBAC deficiency, we pave the way for novel therapeutic strategies aimed at restoring immune homeostasis,” said Oda, the lead author of the study.
He added, “One of the patients with SHARPIN deficiency had been dependent on a wheelchair for years before we first saw him. His ankles were inflamed, and it was too painful to walk. The genetic diagnosis enabled us to target the right molecular pathway underlying his conditions.”
Since being placed on anti-TNF therapies, the patient has been symptom-free for almost seven years. “As both a clinician and a scientist, I am thrilled to have the opportunity to positively impact one patient’s life through our research,” Oda concluded.
More information:
Hirotsugu Oda et al, Biallelic human SHARPIN loss of function induces autoinflammation and immunodeficiency, Nature Immunology (2024). DOI: 10.1038/s41590-024-01817-w
Citation:
Protein responsible for genetic inflammatory disease identified (2024, April 29)
protein-responsible-genetic-inflammatory-disease.html
.
. The content is provided for information purposes only.
