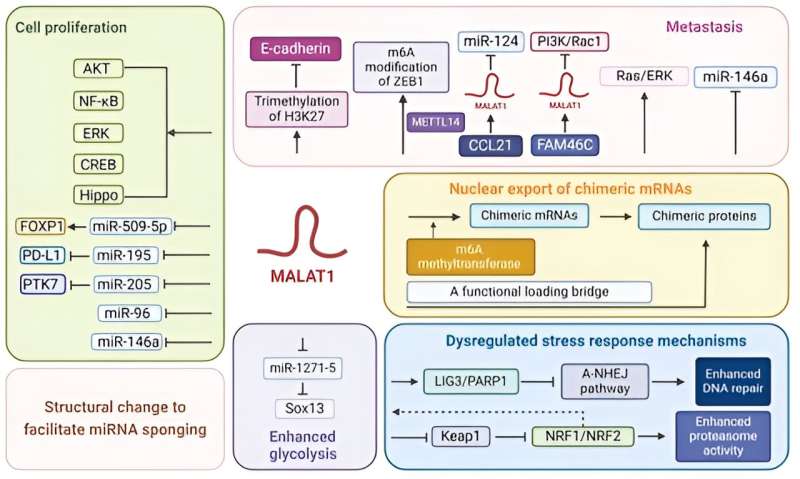
Long non-coding ribonucleic acids (lncRNAs), despite not coding for any protein, can play a significant role in cancer formation, progression, and treatment outcomes. One such lncRNA of interest is MALAT1, abbreviated for metastasis-associated lung adenocarcinoma transcript 1.
Initially noted for its impact on gene expression in non-small-cell lung cancer cells, MALAT1’s overexpression has been linked to increased metastasis risk and poor treatment outcomes. Metastasis is the ability of cancer cells to spread from the original site to other regions.
The overexpression of MALAT1 has been detected in various cancers, such as breast, cervical, and gastric cancers. In a new study, a team of researchers led by Professor Heng Xu and Professor Yang Shu from West China Hospital, Sichuan University, China, delves deeply into the role of MALAT1 in blood cancer formation, metastasis, stress response, and metabolic changes.
Based on the results from pioneering preclinical studies, the review also investigated MALAT1’s potential as a therapeutic target for blood cancer. This study was published online in the Chinese Medical Journal on April 1.
When asked about the motivation behind this review, Prof. Xu says, “In addition to solid cancers, increasing evidence in recent years has also established a potential role of MALAT1 in hematological malignancies, but a systematic review is lacking. Therefore, herein we present an updated, comprehensive overview that highlights various functions of MALAT1 in hematological malignancies.”
The review highlighted the overexpression of MALAT1 in different blood cancers, including multiple myeloma (cancer of the plasma cells in the bone marrow), lymphoma (cancer of the disease-fighting lymphatic system), and leukemia (cancer of the blood-forming tissues). Studies investigating MALAT1 in various hematological malignancies consistently show that elevated MALAT1 levels correlate with adverse clinical outcomes.
Additionally, these studies also showed that MALAT1 plays a crucial role in regulating various aspects of blood cancer cells, including proliferation, migration, invasion, metastasis, stress response, nuclear export of abnormal messenger RNA called chimeric mRNA, and energy generation through metabolic pathways like glycolysis. Consequently, MALAT1 induces structural changes in these cancer cells.
Specifically, MALAT1 promotes blood cancer cell proliferation by acting as competitive endogenous RNA, which interferes with the activity of specific microRNAs that suppress cancer-causing pathways. Studies also show that MALAT1 suppresses an essential type of cell death, called apoptosis, in cancer cells.
Additionally, MALAT1 facilitates blood cancer cell migration, invasion, and metastasis by promoting epithelial-mesenchymal transition—an event where stationary epithelial cells convert into mobile mesenchymal cells, which, in turn, allows cancer cells to spread from the original site to other body parts. Furthermore, studies have shown that MALAT1-aided nuclear export of chimeric mRNAs could sustain leukemia.
In addition, multiple studies have shown that MALAT1 contributes to chemotherapeutic drug resistance in blood cancer through various mechanisms. MALAT1 could bolster cancer cell deoxyribonucleic acid (DNA) repair and cell cycle progression. Concurrently, MALAT1 could reduce chemotherapeutic drug uptake and even degrade these drugs.
Because of MALAT1’s significant role in promoting blood cancer, researchers highlight its potential as an effective therapeutic target. Preclinical studies using laboratory-synthesized biomolecules, called small interfering RNA (siRNA) and antisense oligonucleotides (ASO), have shown promising outcomes in halting cancer cell growth and prolonging survival in mice with blood cancer.
Prof. Xu adds, “However, a clinical study of using siRNA or ASO to target MALAT1 is currently lacking. Toxicological and toxicokinetic studies in non-human primates and subsequent clinical trials are still required to evaluate the safety of these treatment strategies.”
Furthermore, MALAT1 levels in the blood reflect disease status and predict treatment response, offering a non-invasive tool for managing blood cancer.
In summary, this review portrays MALAT1 as a significant player in different types of blood cancer, impacting various aspects of cancer progression. While the potential of MALAT1 in assessing the status and treating such cancer is evident, researchers highlight the need for further studies to translate these findings into effective real-world treatments, offering hope of improved outcomes for many patients globally.
More information:
Chunlan Zhang et al, Long non-coding RNA MALAT1 in hematological malignancies and its clinical applications, Chinese Medical Journal (2024). DOI: 10.1097/CM9.0000000000003090
Provided by
Cactus Communications
Citation:
Study reviews the role of a ribonucleic acid ‘MALAT1’ in hematological malignancies (2024, April 24)
role-ribonucleic-acid-malat1-hematological.html
.
. The content is provided for information purposes only.
